ケーススタディー
We began our relationship with Saranas back in 2015, as the Texas-based start-up set out on its mission to improve patient outcomes by detecting internal bleeding complications early. Half a decade on, we’re proud of our contribution to its Early Bird Bleed Monitoring System, granted de novo classification by the United States Food and Drug Administration (FDA) as the first and only system in the world for early bleed detection during endovascular procedures.

Driving efficiency and creativity
We worked hard to make sure our input into the project was characterized by smooth cooperation and a positive spirit of teamwork from the get-go. Saranas chose to partner with us because of our unique ability to offer key capabilities and disciplines under the same roof. Our design, human factors and mechanical engineering teams collaborated seamlessly to drive efficiency and creativity, while retaining the integrity of the original concept.

Vascular access sheath
Saranas’s objective was to measure changes in bioimpedance to detect and monitor bleeding from vessel injury during endovascular procedures, such as a transcatheter aortic valve replacement (TAVR). Visual and audible indicators would notify the clinician of the onset and progression of a bleed. This would occur when a vascular access sheath with embedded sensors detects changes in electrical resistance across the blood vessel.
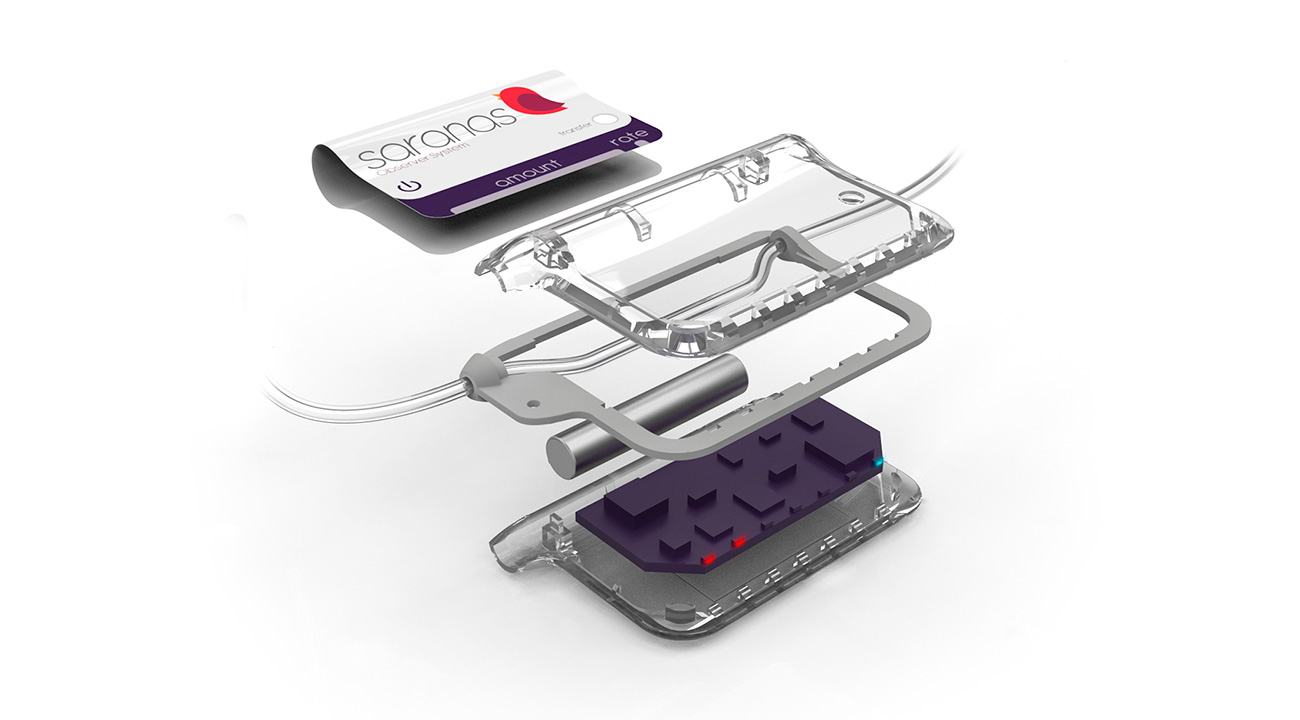
System concept
We developed the system concept to include a sheath that connects to a dongle device which is positioned along its flush line. The device contains a battery, a PCB and a user interface. We used an analysis of the system from physics first principles to identify a performance envelope and recommend an approach for the detection algorithm.
課題
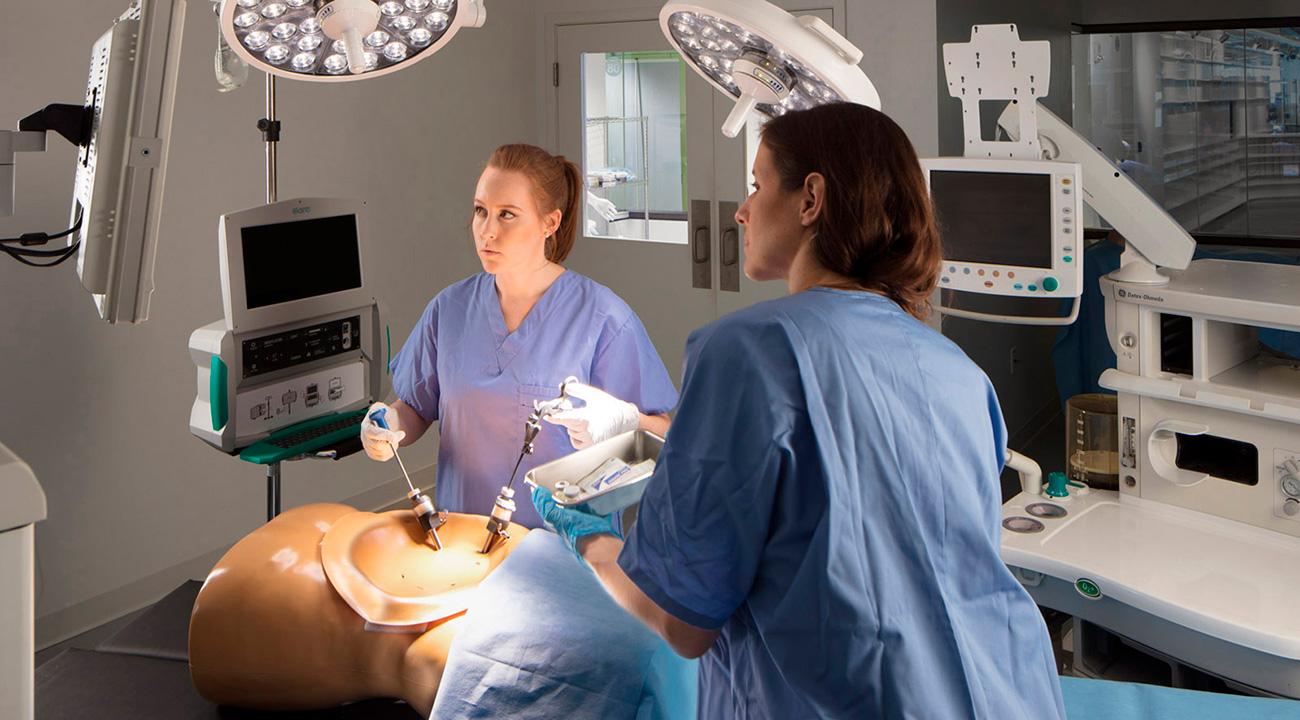
Putting user needs first
The key challenge in the early stages was to decide how best to present the data that the electrodes on the sheath were recording. Our brainstorming of potential wireless connectivity took us on to Human Factors and a firm decision to put user needs first and let them guide us. That meant framing the design challenge from a holistic point of view, with special focus on feasibility, usability, desirability and viability.

Accelerating time to market
Experience told us that there would be no point delivering a design with the necessary hardware and software functionality if clinicians found it inelegant and obtrusive. We also knew that surgeons would be naturally resistant to an over-emphasis on data. It led to a crucial recommendation, supported by Saranas, to avoid weighing the device down with heavy Bluetooth data connectivity. This classic Minimal Viable Product strategy streamlined the design process and accelerated time to market.

Complex connectivity
Collaboration came to the fore once again in the shape of a smooth three-way partnership between us, Saranas and Vention Medical. Now part of Nordson Medical, they developed the sheath component of the monitoring system. We were able to rise to a considerable design challenge to ensure that the complex connectivity of the sheath, fluid line and device interface was effective.
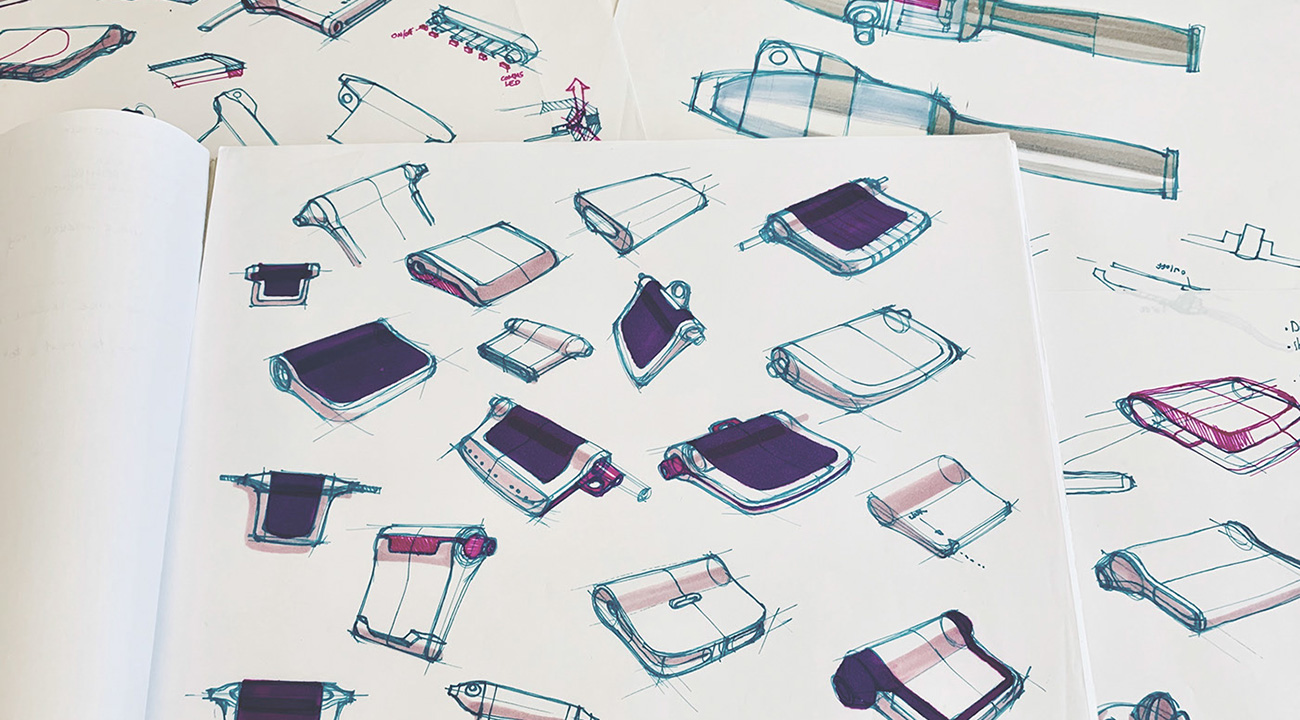
Driving creative vision
The look and feel of the device were developed simultaneously with the technology. Industrial design was considered in tandem with graphic design. Both were shaped by the driving creative vision for the device to appear like a simple ‘product label’ tagged elegantly to the flush line tubing. A sleek bulge would house the battery. The body was given a subtle triangular shape to ensure visibility of the LED user interface display, regardless of orientation.

Journey to launch
As the form factor was created and the connected system became embodied, the team went to work on typography, color palette and brand identity. The design integrity – focused on technical and commercial goals – was retained throughout the journey to launch, and eventually to the first commercial use of the Early Bird Bleed Monitoring System in the United States.